Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 15, Article number: 39656 (2025)
1584
Metrics details
Simiao Biejia Decoction (SMBJ), a traditional Chinese herbal formula, has been clinically applied to treat diabetes mellitus (DM)-induced male dysfunction due to its kidney-tonifying, heat-clearing, and blood-enriching properties. However, the precise molecular mechanisms and therapeutic targets through which SMBJ confers protection against DM-induced testicular damage remain to be fully elucidated. Goto-Kakizaki (GK) and wild-type rats were randomly assigned to the wild-type, GK model, and SMBJ low-, medium-, and high-dose groups. SMBJ was administered at low, medium, and high doses (2.44, 4.88, and 9.76 g/kg/day) by gavage for 4 weeks. Serum testosterone, blood glucose, and oxidative stress levels were measured using ELISA and colorimetric assays. Histopathological staining was performed to evaluate testicular damage. In vitro experiments were conducted using the R2C cell line subjected to high-glucose conditions, with intervention using SMBJ-containing serum. RT-qPCR, Western blotting, immunofluorescence, MeRIP-qPCR, RNA stability were employed to elucidate the underlying mechanisms. SMBJ alleviates oxidative stress injury, thereby reducing apoptosis and enhancing testosterone secretion in the interstitial tissue and in high glucose-stimulated R2C cell of diabetic rats. METTL3 expression was downregulated in the testes of diabetic rat and in R2C cell exposed to high glucose. METTL3 knockdown mimicked the effects of high glucose, underscoring its protective role in Leydig cells. Si-METTL3 decreased the stability of PI3K/AKT mRNAs, whereas SMBJ upregulated METTL3 expression, modulating the PI3K/AKT pathway, reversing high glucose-induced damage in R2C cell, and highlighting its therapeutic potential in Leydig cell protection. SMBJ may upregulates METTL3 expression alongside PI3K/AKT activation to ameliorate diabetes mellitus-induced Leydig cell dysfunction. The potential regulatory role of m6A modification in diabetes-induced testicular injury was further elucidated.
Diabetes mellitus(DM) is a chronic metabolic disorder, and the global prevalence rate is gradually increasing and becoming a major threat to human health, with type 2 diabetes mellitus (T2DM) accounting for 90–95% of patients with diabetes1. Diabetes mellitus has significant effects on the reproductive system, including hypothalamic‒pituitary‒gonad (HPG) axis dysfunction, testosterone synthesis and secretion reduction, testicular failure, spermatogenesis disorders, erectile dysfunction (ED) and ejaculatory disorders. The incidence of secondary functional hypogonadism has significantly increased2,3. The damaging effects of diabetes on tissues are attributed to oxidative stress caused by hyperglycaemia, and excessive glucose levels of reactive oxygen species (ROS) occur due to high levels of reactive oxygen species in the blood. In general, high blood sugar and lipid imbalances trigger a series of inflammatory pathways that lead to testicular destruction and dysfunction as well as reproductive disorders4,5. Testosterone is synthesized mainly by Leydig cells in the testis6. A long-term high-glucose environment destroys the balance of antioxidant capacity in cells, promotes an increase in mitochondrial ROS production, and then triggers Leydig cell oxidative stress7.Oxidative stress, inflammatory reactions and apoptosis are detected in the testes of some patients with diabetes. In particular, T2DM is associated with decreased serum testosterone levels and clinical symptoms of hypogonadism in men over 30 years of age8,9,10. Jiang et al. determined the effect of T2DM on testosterone levels through Mendelian randomization studies and reported that oral metformin and exogenous insulin injection improved the low testosterone status of patients with diabetes after adjusting for common treatment methods11.
Simiao Biejia Decoction (SMBJ) is based on Simiao Yongan Decoction and Erdi Biejia Decoction. Its components include turtle shell, Salvia miltiorrhiza, Angelica sinensis, Radix Glycyrrhizae, Radix Puerariae, Rhizoma Coptidis, Astragalus membranaceus, Flos Lonicerae, Litchi seed, Radix Rehmanniae Preparata, Radix Scrophulariae, Fructus Lycii and Epimedium. From the point of view of TCM, “yin deficiency internal heat” and “qi stagnation and blood stasis” usually comprise the basic pathology and pathogenesis of DM. SMBJ nourishes yin, clears heat, regulates qi and enriches blood.
Methyltransferase like-3 (METTL3) is a major methyltransferase responsible for m6A modification and targeting of mRNA stability12,13,14. METTL3 plays roles in various pathophysiological processes, including the cell cycle, apoptosis, innate immunity and inflammation, as well as cell proliferation, migration, invasion and differentiation14,15. Jiang et al.demonstrated that PM2.5 exposure induces METTL3-mediated m6A methylation in Leydig cells, followed by the regulation of autophagy flux via the METTL3–m6A–SIRT1 signalling axis, ultimately resulting in the inhibition of testosterone biosynthesis16. Therefore, we hypothesized that m6A modification in Leydig cells affects Leydig function.
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signalling pathway is the main signalling cascade that promotes cell survival and proliferation17.Elevated blood glucose levels or insulin resistance can suppress the PI3K/AKT pathway, resulting in compromised interstitial cell function, decreased testosterone synthesis, and heightened cell apoptosis. In the presence of oxidative stress, such as an accumulation of reactive oxygen species, AKT mitigates lipid peroxidation damage in interstitial cells by activating antioxidant pathways like nuclear factor E2 related factor 2(Nrf2)18.Wu X et al. confirmed that quercetin improves the ageing of Leydig cells and promotes testosterone synthesis by regulating the AR/PI3K/AKT signalling pathway19.We have also previously confirmed that SMBJ improves diabetes erectile dysfunction (DMED), increases endothelial nitlic oxide synthase(eNOS) and neuronal nitlic oxide synthase(nNOS) levels, reduces oxidative stress levels, and improves angiogenesis and the stability of penis tissue through the PI3K/Akt/nNOS signalling pathway20. However, the mechanism of testicular stromal cell damage induced by SMBJ treatment in diabetes is unclear. Therefore, the aim of this study was to explore the mechanism by which SMBJ improves testosterone secretion from injured testicular stromal cells in diabetic rats through in vitro and in vivo experiments to provide a new direction for the clinical treatment of SMBJ.
The Goto-Kakizaki (GK) rat, a well-characterized nonobese spontaneous model of T2DM, has been widely employed in scientific investigations of diabetes pathogenesis. The GK/Wistar rat strain, a genetically homogeneous rodent model exhibiting spontaneous T2DM characteristics, was utilized as the diabetic cohort, and age-matched wild-type Wistar rats served as the nondiabetic control group. Forty male GK/Wistar rats (14 weeks old, body weight 280–320 g) and 10 male Wistar rats were procured from the Animal Experiment Centre of Cavens in Changzhou, Jiangsu, China. All experimental procedures were approved by the Animal Ethics Committee of Southeast University (Approval No. 20200402003). The combination included 3.3 g of Carapax trionycis, 5 g of Radix salviae liguliobae, 6.7 g of Radix angelicae sinensis, 1.7 g of Radix glycyrrhizae, 4 g of Radix puerariae, 0.7 g of Rhizoma coptidis, 4 g of Radix astragali, 3.3 g of Flos lonicerae, 3 g of Semen litchi, 7.7 g of Radix rehmanniae preparata, 6.6 g of Radix scrophulariae, 8.3 g of Fructus lycii, and 2 g of Epimrdii herba. We obtained the total ion chromatogram of SMBJ through UHPLC‒QTOF‒MS analysis in the early stage and qualitatively identified the compound using Compound Discoverer 3.3 software20. The constituent materials were pulverized to a particle size < 50 μm using a planetary ball mill, followed by dissolution in deionized water to prepare SMBJ suspensions. The suspension was aseptically transferred into sterile polypropylene centrifuge tubes (Corning, USA) and cryopreserved at − 80 °C.
The Leydig cell line R2C was procured from the American Type Culture Collection (ATCC). The cells were maintained in F12 medium (Gibco, USA) supplemented with 10% horse serum (Proteinbio, China), 2.5% FBS (Proteinbio, China) and 1% penicillin/streptomycin (Proteinbio, China). These cells were incubated in a 5% CO2 incubator at 37 °C.
GK rats (n = 40) were randomly divided into GK (positive control), low-dose SMBJ (LSMBJ), medium-dose SMBJ (MSMBJ), and high-dose SMBJ (HSMBJ) groups. Another 10 Wistar rats composed the normal control group. The rats were orally administered SMBJ at three dose levels: LSMBJ (2.44 g/kg), MSMBJ (4.88 g/kg), and HSMBJ (9.76 g/kg). The rats in the GK and control groups were administered saline via oral gavage twice daily. After 4 weeks of SMBJ treatment, testicular tissues were fixed with G1101 (Servicebio, China) overnight at 4 °C, dehydrated, paraffin embedded, and sectioned into 5-µm-thick slices mounted on glass slides for histological analysis. Additional samples were snap-frozen in liquid nitrogen and stored at − 80 °C for further studies.
Preparation of drug containing serum:10 male Wistar rats were purchased from Charles River (Beijing, China).The dosage of SMBJ granules used in the group was 10 times the gavage dose used in the MSMBJ groups. The treatments were administered twice a day for 7 days. The rats were anaesthetized with 10% pentobarbital sodium 2 h after the last administration. Blood was collected from the abdominal aortas and centrifuged at 3000 rpm for 15 min at 4 °C. Serum samples were collected, filtered with 0.22 μm filters, inactivated at 56 °C for 30 min and then stored at − 80 °C. During the cell experiments, different concentrations of drug-containing serum were added at a volume ratio of 1/10 of the culture medium. R2C cell were modelled with high glucose.
Following an overnight fast, fasting blood glucose (FBG) levels were measured using a Contour Next glucometer (Abbott Laboratories, USA).
The hyperglycaemic conditions associated with diabetes may induce structural damage to testicular tissues, particularly in the testicular interstitium, which could impair testosterone secretory function. This study aimed to evaluate morphological alterations, investigate the effects of pharmacological intervention on testicular histology, and elucidate the associated molecular mechanisms. Testicular tissues were immediately fixed in 4% paraformaldehyde (G1101; Servicebio, China) at 4 °C, sequentially processed through dehydration, paraffin embedding, and sectioning into 5-µm-thick slices mounted on polylysine-coated glass slides. After dewaxing with xylene, the sections were stained with haematoxylin and eosin (H&E; G1104, Servicebio Technology, Wuhan, China) for histopathological evaluation.
In brief, the testicular tissue was homogenized and centrifuged in a glass Teflon homogenizer with precooled physiological saline at 3000 ×g for 15 min at 4 °C. Testicular tissues were homogenized in lysis buffer and centrifuged at 12,000 ×g for 10 min at 4 °C to collect the supernatants. Oxidative stress markers, including malondialdehyde (MDA) and superoxide dismutase (SOD) activities, were quantified using commercially available assay kits according to the manufacturer’s protocols.
Small interfering RNA (siRNA) targeting METTL3 (si-METTL3, si1, forwards: 5’-GUCUAUAGUCCCUGAAUUAGC-3’ and reverse: 5’-UAAUUCAGGGACUAUAGACGU-3’; si2, forwards: 5’-CAGUGGAUCUGUUGUGAUATT-3’ and reverse: 5’-UAUCACAACAGAUCCACUGTT-3’; si3, forwards: 5’-GGAUUGCGAUGUGAUUGUATT-3’ and reverse: 5’-UACAAUCACAUCGCAAUCCTT-‘′), and their negative control (NC, forwards: 5’-UUCUCCGAACGUGUCACGUTT-3’ and reverse: 5’-ACGUGACACGUUCGGAGAATT-3’) were purchased from Ruizhen Biology (NanJing, China). Lipo8000™ transfection reagent (Beyotime, China) was used for siRNA transfection into R2C cell. Briefly, R2C cell were cultured in plates with penicillin/streptomycin-free medium. The transfection reagent and siRNA (20 µM) were dissolved in DMEM and mixed for 20 min before being added to the culture plate.
RNA extraction was performed using TRIzol reagent (Vazyme, China), followed by quantification of the RNA concentration and purity assessment using a NanoDrop 2000 (Thermo Fisher, USA). cDNA synthesis was subsequently carried out using PrimeScript RT Master Mix (TaKaRa, Japan) with 1 µg of total RNA. The following conditions were used for cDNA amplification: initial denaturation for 30 s at 95 °C, followed by 40 cycles for 30 s at 95 °C and 60 s at 60°C. A single product was determined to be amplified through melt curve analysis. The 2-ΔΔCT method was used to calculate the relative target gene mRNA abundance. The primers used in our study were as follows: PI3K, forwards: 5’-TCATGGATGCTTTGCAGGGT-3’, and reverse: 5’-TGCCGTAAGTCATCGCCATT-3’; AKT, forwards: 5’-TATGAGAAGAAGCTGAGCCCAC-3’, and reverse: 5’-CACACTCCATGCTGTCATCTT-3’;TNF-α, forwards: 5’-CCAACAAGGAGGAGAAGTTCC-3’′, and reverse: 5’-CTCTGCTTGGTGGTTTGCTAC-3’; Nrf2, forwards: 5’-GACCTAAAGCACAGCCAACACAT-3’, and reverse: 5’-CTCAATCGGCTTGAATGTTTGTC-3’; and GAPDH, forwards: 5’-TGCCACTCAGAAGACTGG-3’, and reverse: 5’-TTCAGCTCTGGGATGACCTT-3’.
Testicular tissues and cells were homogenized in RIPA lysis buffer (NCM Biotech, China) containing 1% protease and phosphatase inhibitor cocktail (NCM Biotech, China) at 4 °C for 30 min. Lysates were subsequently centrifuged at 12,000 ×g for 15 min at 4 °C. Protein concentrations were determined using a bicinchoninic acid (BCA) assay kit (Beyotime, China) according to the manufacturer’s instructions. The supernatants were subsequently mixed with 5× SDS‒PAGE loading buffer (Beyotime, China) at a 1:4 volume ratio and heat denatured at 95 °C for 10 min. A total of 30 µg of protein per sample was resolved on 12.5% SDS‒PAGE gels (NCM Biotech, China) under denaturing conditions. Following electrophoretic separation, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, USA) using a semidry transfer system. The membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature, followed by incubation with primary antibodies diluted in blocking buffer at 4 °C overnight, including METTL3 (Proteintech, 15073-1-AP, 1:10000), PI3K (Proteintech, 67071-1-Ig, 1:2000), P-PI3K (Affinity, AF3242, 1:2000), AKT (Cell Signaling, 9272 S, 1:1000), P-AKT (Proteintech, 6444-1-Ig, 1:2000), Bcl-2 (Cell Signaling, 3498 S, 1:1000), Bax (Proteintech, 50599-2-Ig, 1:2000), steroidogenic acute regulatory protein (StAR) (Proteintech, 67130-1-Ig, 1:10000), and β-actin (Proteintech, 66009-1-Ig, 1:20000) antibodies. Goat anti-rabbit IgG (Proteinbio, PA2202, 1:5000) or goat anti-rabbit IgG (Proteinbio, PA2201, 1:5000) was then incubated with the membranes for 1 h at room temperature. Chemiluminescent signals were detected using an ECL solution (Yeasen, China). Band intensities were semiquantitatively analysed using ImageJ software.
A rat testosterone ELISA kit was obtained from Jining Biotechnology (Shanghai, China). Testosterone levels in the samples were measured using the rat testosterone ELISA kit according to the manufacturer’s instructions.
Fixed testicular tissues were paraffin-embedded for histopathological evaluation. The tissue blocks were sectioned into 5 μm slices using a rotary microtome (Leica RM2235, Germany) and mounted on polylysine-coated glass slides. The sections were deparaffinized in xylene (3 × 5 min) followed by rehydration through a descending ethanol series (100%, 95%, 80%, 70%). Antigen retrieval was performed by incubating the slides in preheated sodium citrate buffer (10 mM, pH 6.0) at 95 ℃ for 15 min in a microwave oven. After the samples were cooled to room temperature and rinsed with PBS (3 × 5 min), nonspecific binding sites were blocked with 5% normal goat serum (Boster Biological, China) in PBS for 30 min at 25 ℃. METTL3 (Proteintech, 15073-1-AP, 1:200), TNF-α (Proteintech, 60291-1-Ig; 1:200), and Nrf2 (Proteintech, 80593-1-RR; 1:300) were incubated with the sections overnight at 4 °C. The secondary antibodies were incubated with the tissue sections for 1 h at room temperature on the following day. A DAB Kit (CWBIO, CW2069, China) was used for DAB-H2O2 staining. Finally, the tissue sections were photographed under a microscope after they were sealed with neutral gum.
Tissue sections and cell monolayers were prepared as previously described. Apoptotic cells in both the tissue sections and the cell monolayers were detected using a TUNEL BrightRed Apoptosis Detection Kit (Vazyme, China). Fluorescence imaging was performed using a fluorescence microscope.
R2C cell were seeded on coverslips in 12-well plates and cultured until they reached 70% confluence. Paraformaldehyde (4%) was used to fix the cells at 37 ℃ for 30 min, after which they were washed with PBS three times. The cell membranes were subsequently permeabilized by incubation with 0.5% Triton X-100 at 37 ℃ for 20 min, followed by washing with PBS three times. Next, nonspecific antigens were blocked with 5% BSA at 4 ℃ for 1 h, followed by washing with PBS three times. METTL3 (Proteintech, 15073-1-AP, 1:200) was then added, and the mixture was incubated overnight at 4 ℃ in a humid box. The next day, after washing with PBS three times, diluted fluorescent secondary antibodies were incubated at room temperature in the dark for 1 h. Following three washes with PBS, the samples were coverslipped with DAPI-containing antifade mounting medium (Beyotime, China) and incubated in the dark for 5 min to minimize photobleaching. After three washes with PBS, the cells were visualized under a fluorescence microscope.
Total m6A RNA methylation levels were quantified using the EpiQuik™ m6A RNA Methylation Quantification Kit (Colorimetric) (Epigentek, New York, USA), according to the manufacturer’s instructions. Total RNA is immobilized on a plate using a high-efficiency RNA binding solution, and m6A is detected using capture and detection antibodies. Colour development was initiated with developer solution and terminated with stop solution. Absorbance was measured at 450 nm, and m6A levels were calculated based on the standard curve.The amount of m6A is directly proportional to the measured OD value.
Total RNA was extracted using the Trizol method as previously described. Methylated RNA immunoprecipitation (MeRIP) was performed using the EpiQuik™ CUT&RUNm6ARNA Enrichment Kit (Epigentek, Farmingdale, USA) following the manufacturer’s instructions. In brief, 1µM of total RNA was incubated with anti-m6A antibody and affinity beads for 90 min to form RNA–antibody–bead complexes. After washing and purification, enriched RNA fragments were eluted and subjected to RT-qPCR.
Following 24 h of siRNA transfection, transcription was inhibited by adding 1 µg/mL actinomycin D to the culture medium. Cells were then collected at 0, 2, 4, and 6 h post-treatment. Total RNA was extracted using the Trizol method and analyzed by RT-qPCR to assess RNA degradation over time.
Statistical analysis was performed using GraphPad Prism version 9.5 for Windows (GraphPad Software, La Jolla, CA, USA). Data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for multigroup comparisons, and the Tukey’s post-hoc test was used for comparisons between two independent groups. The significance of the difference between the two groups was evaluated by the Student’s t- test, with P < 0.05 being considered as statistically significant.
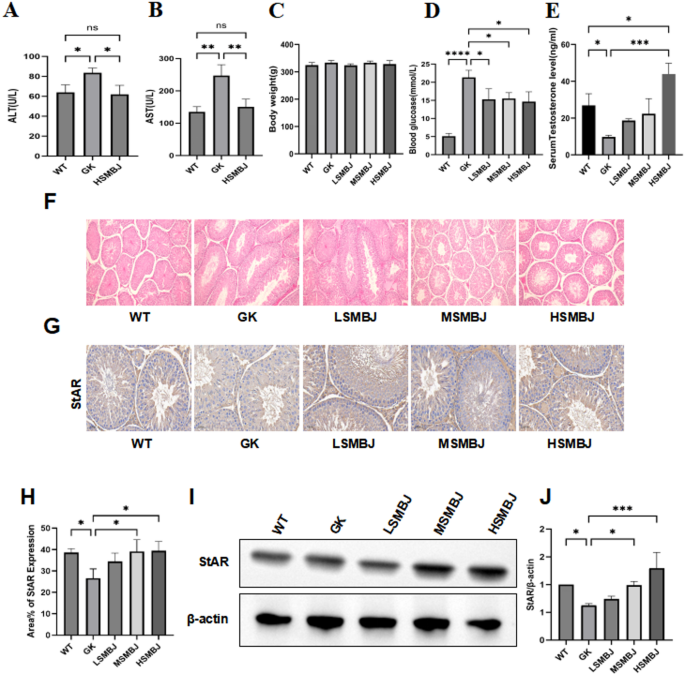
Figure 1 displays the body weight and blood glucose and testosterone levels of diabetic rats at the conclusion of the experiment. As shown, high-dose SMBJ is safe for liver function (Fig. 1A,B).No significant differences in body weight were observed among the groups (Fig. 1C). Blood glucose levels were notably elevated in the GK group compared with those in the control group and decreased after SMBJ treatment (Fig. 1D). Furthermore, the decrease in testosterone levels in GK rats was significantly ameliorated in SMBJ-treated rats (Fig. 1E). These findings indicate that SMBJ may lower blood glucose levels and increase testosterone secretion in the testes. To elucidate the mechanism of SMBJ action, histological analysis was conducted. HE staining revealed that, relative to those in the control group, the testicular stroma in the GK group exhibited substantial atrophy and loose tissue (Fig. 1F), indicating that the hyperglycaemic state of diabetes can induce testicular damage. However, medium and high doses of SMBJ reversed this damage, restoring the testicular stromal tissue structure. To investigate the impact of SMBJ on testosterone production in diabetes mellitus-induced conditions, the expression of StAR was assessed using immunohistochemistry and Western blot analysis. The findings revealed a decrease in StAR protein levels in the GK group, whereas a significant increase was observed in the SMBJ-treated group (p < 0.05) (Fig. 1G–J).
SMBJ improves testicular injury caused by DM. (A) and (B)Liver function indicators ALT and AST. (C) Body weight. (D) blood glucose levels.*P < 0.05,****P < 0.0001. (E) Testosterone levels(n = 3).*P < 0.05, ***P < 0.001.(F)HE staining was performed to show pathophysiological changes in the testicles. . (G) and (H) Immunohistochemistry (IHC) of StAR(n = 3).*P < 0.05. (I) and (J) The western blot results of testicular tissue StAR, and data shown are mean ± standard deviation(n =3). *P < 0.05, ***P < 0.001.
Testicular tissue, characterized by a high metabolic rate, is particularly vulnerable to reactive oxygen species and oxidative stress21. Hence, this study investigated the influence of SMBJ on testicular oxidative stress. Immunohistochemical analysis revealed a significant decrease in TNF-α expression and an increase in Nrf2 expression following SMBJ treatment compared with those in the GK group (Fig. 2A–D). Furthermore, SMBJ treatment effectively normalized the MDA and SOD levels in testicular tissue. Compared with those of the control group, colorimetric assessment revealed a significant increase in MDA expression and a decrease in SOD levels in the testicular tissue of GK rats. Conversely, following SMBJ treatment, MDA levels were notably reduced, and SOD levels were elevated compared with those in the GK group (Fig. 2E,F).
SMBJ improves oxidative stress injury in the testis induced by DM. (A) and (C) Immunohistochemistry (IHC) of TNF-α (n = 3). *P < 0.05, **P < 0.01. (B) and (D) Immunohistochemistry (IHC) of Nrf2 (n = 3). *P < 0.05, **P < 0.01. (E) Malondialdehyde (MDA) levels. The data shown are the means ± standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (F) Superoxide dismutase (SOD) activity. The data shown are the means ± standard deviations(n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
SMBJ markedly ameliorated diabetes-induced apoptosis, as evidenced by TUNEL fluorescence staining, revealing a significant increase in fluorescence intensity in the GK group compared with the control group, which was significantly reversed by SMBJ treatment (Fig. 3A,B). The Western blot results corroborated the TUNEL staining findings, showing an elevated Bax/Bcl-2 ratio in the GK group and a decreased ratio in the SMBJ treatment group (Fig. 3C,D), suggesting that hyperglycaemia triggers apoptosis and that SMBJ effectively counteracts this process.
SMBJ improves testicular interstitial apoptosis induced by DM. (A) and (B) TUNEL assay of testicular tissue. (C) and (D) Western blot results of Bax/Bcl2 in testicular tissue from the five groups. The data shown are the means ± standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
We previously conducted a study to investigate the potential involvement of the METTL3/PI3K/AKT pathway in the therapeutic effects of SMBJ on diabetes-induced interstitial damage in rats. Western blot analysis was employed to evaluate the level of PI3K/AKT and its phosphorylation status. Compared with the normal control group, the GK group presented significantly lower p-AKT1/AKT1 protein levels in the testis (p < 0.05). Compared with GK treatment, SMBJ treatment led to notable increases in p-PI3K/PI3K and p-AKT1/AKT1S protein levels in testicular tissue (p < 0.05) (Fig. 4A–C,F). Furthermore, Western blot and immunohistochemical analyses revealed a significant reduction in METTL3 protein expression in the testicular tissue of the GK group (p < 0.05), whereas SMBJ treatment resulted in a marked increase in METTL3 protein expression in the testicular tissue compared with those in the GK group (p < 0.05) (Fig. 4D,E).
SMBJ may improve testicular interstitial injury caused by diabetes through the METTL3/PI3K/AKT pathway. (A), (B), (C) and (F) Results of Western blot analysis of METTL3, P-AKT/AKT, and P-PI3K/PI3K expression in testicular tissue from the five groups. The data shown are the means ± standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (D) and (E) Immunohistochemistry (IHC) of METTL3 (n = 3). *P < 0.05, **P < 0.01.
To determine whether SMBJ promotes Leydig cell functional recovery by regulating METTL3 expression, R2C cell were transfected with METTL3 small interfering RNA (siRNA) and subsequently treated with SMBJ.The efficiency of METTL3 siRNA was confirmed by Western blot analysis(Fig. 5A,B). High-glucose modelling was subsequently performed, and a CCK8 assay revealed that different concentrations of high glucose slowed the proliferation of R2C cell. At 48 h, 30 mmol/L high glucose had the greatest effect on the cells (p < 0.05) (Fig. 5C). Moreover, high-glucose treatment slowed the proliferation of R2C cell, and SMBJ improved this effect in a dose-dependent manner (p < 0.05) (Fig. 5D). Western blot analysis further demonstrated that SMBJ significantly counteracted high glucose-induced molecular changes and reversed the effect of METTL3 knockdown on R2C cell. Specifically, it reversed the upregulation of PI3K, AKT and and their phosphorylation (Fig. 5E–H). These results support the conclusion that SMBJ upregulates METTL3 expression alongside PI3K/AKT activation.To validate the molecular mechanism, we next examined the effect of METTL3 knockdown on m6A RNA methylation.A colorimetric m6A quantification assay revealed a significant reduction in total m6A levels following METTL3 siRNA(Fig. 5I).MeRIP-qPCR assays demonstrated decreased m6A enrichment on PI3K and AKT mRNA in cells knocking down METTL3(Fig. 5J,K).We next investigated the effect of METTL3 knockdown on the stability of PI3K and AKT mRNAs by measuring their levels at 2, 4, and 6 h following treatment with actinomycin D (1µM). RNA stability assays confirmed that METTL3 knockdown decreases the half-life of PI3K/AKT mRNAs (Fig. 5L,M).These findings suggest that METTL3 may enhance PI3K/AKT pathway activity by stabilizing its mRNA transcripts in R2C cell.
SMBJ improves Leydig cell function by upregulating METTL3 and affecting the PI3K/AKT pathway. (A) and (B) METTL3 was successfully knockdown using small interfering RNA. The data from the Western blot analysis of METTL3 are presented as the means ± standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) The effect of different concentrations of high glucose on R2C cell, and (D) The effect of different concentrations of SMBJ on R2C cell (L:10 µl/ml, M:50 µl/ml, H:100 µl/ml). (E), (F), (G) and (H) METTL3 knockdown attenuated the upregulation of METTL3 caused by SMBJ at the protein level. Western blot results for METTL3, P-AKT/AKT, and P-PI3K/PI3K. The data shown are the means ± standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (I) Total m6A levels following METTL3 knockdown in R2C cell (n = 3). (J) MeRIP-qPCR showing reduced m6A modification in PI3K mRNA upon METTL3 knockdown (n = 3). (K) MeRIP-qPCR showing reduced m6A modification in AKT mRNA upon METTL3 knockdown (n = 3). (L) and (M) Stability of PI3K/AKT mRNA after the inhibition of transcription by actinomycin D.
Following high-glucose modelling and METTL3 knockdown in R2C cell, TUNEL fluorescence revealed an increase in METTL3 expression, which was subsequently reversed by SMBJ treatment (Fig. 6A,B). Western blot analysis revealed significant increases in the Bax/Bcl2 ratio in both the Glu and Glu + si-METTL3 groups, which were reduced after SMBJ treatment. Moreover, compared with the SMBJ group, the SMBJ + si-METTL3 group presented a significant increase in the Bax/Bcl2 ratio (p < 0.05), confirming the pronounced effect of SMBJ on cell state recovery(Fig. 6C,D). ROS detected by DCFH-DA staining(Fig. 7E).The fluorescence intensity showed a significant increase in ROS levels in the Glu and Glu + si-METTL3 groups, while the control group and SMBJ group had lower levels(Fig. 6F).Compared with those in the control group, the levels of MDA and SOD in the Glu and Glu + si-METTL3 groups were significantly elevated, whereas SOD expression was decreased. Following SMBJ treatment, the MDA and SOD levels returned to normal (p < 0.05) (Fig. 6G,H). The q-PCR results revealed a significant increase in TNF-α mRNA levels and a decrease in Nrf2 mRNA levels in the Glu and Glu + si-METTL3 groups compared with those in the control group. SMBJ treatment restored these levels to normal levels (p < 0.05) (Fig. 6I,J). Compared with the SMBJ group, the SMBJ + si-METTL3 group presented similar differences, suggesting that METTL3 knockdown promoted oxidative stress in R2C cell, whereas SMBJ ameliorated oxidative stress damage.
SMBJ improve oxidative stress and apoptosis of testicular interstitial cells by upregulating METTL3. (A) and (B) Tunel of R2C cell. (C) and (D) The western blot results of Bax/Bcl2, and data shown are mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (E) and (F) Representative fluorescence images of ROS detected by DCFH-DA staining. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (G) The levels of malondialdehyde (MDA) (n = 3). Data shown are mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (H) The activities of superoxide dismutase (SOD). Data shown are mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (I) The q-PCR of TNF-α mRNA expression (n = 3).*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (J) The q-PCR of Nrf2 mRNA expression (n = 3).*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To further explore whether the METTL3/PI3K/AKT pathway mediates the effects of SMBJ on R2C cell function, we first examined StAR protein expression and testosterone secretion in R2C cell under different treatments. The results revealed that StAR protein expression in the Glu group decreased, but that in the SMBJ group increased. Furthermore, StAR expression decreased following METTL3 knockdown in R2C cell (p < 0.05) (Fig. 7A,B). Testosterone levels, as determined by ELISA in R2C cell, were lower in the Glu and Glu + si-METTL3 groups than in the control group, whereas testosterone levels were increased after SMBJ treatment (p < 0.05) (Fig. 7C). Next, we investigated whether the PI3K/AKT pathway was involved in this process. As shown in Figures D–F, inhibition of PI3K with LY294002 in si-METTL3 cells further suppressed StAR expression and testosterone secretion. However, co-treatment with SMBJ partially reversed these inhibitory effects, although the recovery was weaker compared to SMBJ treatment alone.These findings indicate that SMBJ improves R2C cell function primarily through the METTL3/PI3K/AKT axis, thereby promoting testosterone synthesis.
SMBJ promotes R2C testosterone secretion. (A) and (B) The western blot of StAR in R2C cell treated with Glu, si-METTL3, SMBJ and data shown are mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (C) R2C cell testosterone levels (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (D) and (E) The western blot of StAR in R2C cell treated with si-METTL3, SMBJ, PI3K inhibitor LY294002 and data shown are mean ± standard deviation(n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (F) R2C cell testosterone levels treated with si-METTL3, SMBJ, PI3K inhibitor LY294002 (n = 3). *P < 0.05, ***P < 0.001.
Diabetes impairs Leydig cell function through multiple mechanisms and ultimately leads to male reproductive endocrine disorder22.Long-term hyperglycaemia induces oxidative stress, promotes excessive generation of ROS, destroys cell membranes and key enzymes involved in androgen synthesis (such as StAR), and inhibits testosterone synthesis23.Hypogonadism, sexual dysfunction and infertility may develop clinically; therefore, effective treatments for Leydig damage caused by diabetes mellitus need to be explored. In this study, we investigated the molecular mechanism by which SMBJ ameliorates Leydig damage induced by diabetes mellitus24,25,26.SMBJ, an herbal formula comprising 13 Chinese herbs, contains luteolin, a natural bioactive compound known for its medicinal properties. Through network pharmacology and proteomics combined with mass spectrometry analysis of Chinese herbs, luteolin has been shown to exhibit anti-inflammatory and antioxidant effects27. Key components of SMBJ, including Radix Rehmanniae Praeparata, Flos Lonicerae, Radix Salviae Miltiorrhizae, Herba Epimedium, Litchi Seed, and Radix Puerariae, demonstrate multitarget and multipathway pharmacological profiles. These components primarily modulate metabolic and inflammation-related disorders by engaging pathways such as Nrf2/heme oxygenase-1(HO-1) and extracellular signal-regulated kinase 1/2(ERK1/2)-Nrf2 for antioxidative effects and nuclear factor-κB(NF-κB), mitogen-activated protein kinase(MAPK), and PI3K/Akt for anti-inflammatory and metabolic regulation, leading to hypoglycaemic, antiobesity, and antiatherosclerotic outcomes. Additionally, SMBJ exhibits organ-protective effects on the heart, liver, and nervous system, along with immune-regulatory properties28,29,30,31,32,33,34. The synergistic actions of these herbal ingredients offer a theoretical foundation for addressing complex conditions such as diabetes and cardiovascular diseases, underscoring the potential of traditional herbs in modern multitarget drug development35.
Research has assessed the influence of SMBJ on the testicular interstitium and its potential mechanism of action. Our results revealed that hyperglycemia can induce oxidative stress in Leydig cells which disrupt mitochondrial homeostasis by triggering conformational activation of the pro-apoptotic protein Bax, inhibiting the anti-apoptotic function of Bcl-2, thereby initiating apoptosis.This oxidative stress-driven apoptosis impairs testosterone secretion, a condition ameliorated by SMBJ treatment. Furthermore, SMBJ was found to increase the protein level of METTL3, a crucial enzyme involved in m6A methylation in R2C cell, and to activate the PI3K/AKT pathway. METTL3 knockdown increased R2C cell apoptosis and attenuated the synergistic impact of SMBJ on the PI3K/AKT pathway, indicating that activation of the METTL3/PI3K/AKT pathway mitigates oxidative stress–induced Leydig cell injury.
N6-methyladenosine (m6A) modification, one of the most prevalent forms of posttranscriptional RNA modification, plays a fundamental role in regulating gene expression and cellular function in various germ cells through the coordinated action of m6A regulatory factors36,37,38,39.METTL3, a principal m6A methyltransferase, is critical for catalyzing this modification and thereby modulating RNA stability, splicing, and translation35.Emerging evidence further indicates that dysregulation of METTL3-mediated m6A modification is closely associated not only with the pathogenesis of diabetes but also with the development of its complications, suggesting that alterations in RNA methylation may contribute to diabetes-induced cellular dysfunction40,41.Liu et al. reported that high-glucose exposure downregulates METTL3 expression in podocytes, resulting in reduced m^6A modification of PTEN and subsequent PTEN upregulation, which in turn suppresses the PI3K/Akt pathway and promotes podocyte inflammation through activation of the NLRP3 inflammasome42.In contrast, Xu et al. observed in high glucose–treated renal tubular HK2 cells that both METTL3 and METTL14 expression were downregulated; however, only METTL14 overexpression significantly increased PTEN levels, thereby inhibiting PI3K/Akt signaling and attenuating HDAC5-mediated epithelial–mesenchymal transition (EMT)43. These findings indicate that the regulatory roles of METTL3 in the PI3K/Akt pathway are likely to be cell type–specific and context dependent. Simiao Biejia may influence diabetes-induced Leydig apoptosis by modulating METTL3 expression.Under high glucose conditions, METTL3 is downregulated; however, SMBJ treatment upregulates METTL3 and enhances the anti-apoptotic activity and testosterone secretion functions of R2C cell. Therefore, it can be inferred that SMBJ may enhance R2C function by modulating METTL3.
Investigation of the mechanism by which SMBJ induces changes in Leydig cell function revealed increased PI3K/AKT levels and phosphorylation in testicular tissue and R2C cell treated with SMBJ. Activation of the PI3K/AKT pathway can counteract cell apoptosis by inhibiting oxidative stress. Furthermore, Si-METTL3 inhibits the activation of the PI3K/AKT signaling pathway and affects the testosterone secretion function of R2C cell. PI3K blockade by LY294002 in si-METTL3–treated cells further diminished StAR levels and testosterone secretion.The regulation of R2C cell can be mediated by METTL3 through the PI3K/Akt signalling pathway(Fig. 8). Consequently, it can be concluded from this study that Simiao Biejia can impact the function of Leydig cells via the METTL3/PI3K/AKT pathway. However, importantly, this study did not investigate the downstream target genes of METTL3. Therefore, future research should concentrate on identifying genes controlled by METTL3 to elucidate the underlying mechanism comprehensively.
Mechanism of SMBJ regulating MTEEL3/PI3K/Akt signaling pathway to improve testosterone production in Leydig cells.
SMBJ improved the Leydig injury and secretion function induced by diabetes through upregulates METTL3 expression alongside PI3K/AKT activation.
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Protein kinase B
Erectile dysfunction
Diabetes erectile dysfunction
Epithelial–mesenchymal transition
Endothelial nitlic oxide synthase
Goto-Kakizaki
Heme oxygenase-1
Hypothalamic‒pituitary‒gonad
Mitogen-activated protein kinase
Malondialdehyde
Methylated RNA immunoprecipitation
Methyltransferase-like-3
Neuronal nitlic oxide synthase
N6-methyladenosine
Nuclear factor E2 related factor 2
Phosphatidylinositol 3-kinase
Phosphatase and tensin homologue
Reactive oxygen species
Small interfering RNA
Simiao Biejia
Superoxide dismutase
Steroidogenic acute regulatory protein
Type 2 diabetes mellitus
He, Z. et al. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. Vivo 35 (5), 2503–2511 (2021).
Article CAS Google Scholar
Huang, R. et al. Diabetes-induced male infertility: potential mechanisms and treatment options. Mol. Med. 30 (1), 11 (2024).
Article CAS PubMed PubMed Central Google Scholar
Raghuraman, R. et al. Male sexual dysfunction and hypogonadism in young adults with type 2 diabetes mellitus: A cross sectional study. J. Hum. Reprod. Sci. 17 (3), 170–177 (2024).
Article CAS PubMed PubMed Central Google Scholar
Tian, Y. et al. N6-Methyladenosine methyltransferase METTL3 alleviates Diabetes-Induced testicular damage through modulating TUG1/Clusterin axis. Diabetes Metab. J. 47 (2), 287–300 (2023).
Article PubMed PubMed Central Google Scholar
Liu, Y. et al. Metformin ameliorates testicular damage in male mice with Streptozotocin-Induced type 1 diabetes through the PK2/PKR pathway. Oxid. Med. Cell. Longev. 2019, p5681701 (2019).
Article Google Scholar
Liang, J. et al. Lycium barbarum Glycopeptide Promotes Testosterone Synthesis and Glucose Metabolism in Leydig Cells of the Testis. Biomolecules https://doi.org/10.3390/biom15030425 (2025).
Article PubMed PubMed Central Google Scholar
Chen, H. et al. Effect of photobiomodulation on CCC-ESF reactive oxygen species steady-state in high glucose mediums. Lasers Med. Sci. 36 (3), 555–562 (2021).
Article MathSciNet CAS PubMed Google Scholar
Putta, S. et al. Diabetes mellitus and male aging: pharmacotherapeutics and clinical implications. Curr. Pharm. Des. 23 (30), 4475–4483 (2017).
Article CAS PubMed Google Scholar
Rashid, K. & Sil, P. C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and Endoplasmic reticulum-dependent apoptotic death. Biochim. Biophys. Acta. 1852 (1), 70–82 (2015).
Article CAS PubMed Google Scholar
Wang, Y. et al. Sulforaphane reduction of testicular apoptotic cell death in diabetic mice is associated with the upregulation of Nrf2 expression and function. Am. J. Physiol. Endocrinol. Metab. 307 (1), E14–23 (2014).
Article CAS PubMed Google Scholar
Jiang, C. et al. New evidence for the effect of type 2 diabetes and glycemic traits on testosterone levels: a two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne). 14, 1238090 (2023).
Article PubMed Google Scholar
Guan, X. et al. Roles of METTL3 and NLRP3 in pyroptosis and prospects in SCIRI. Front. Immunol. 16, 1552704 (2025).
Article CAS PubMed PubMed Central Google Scholar
Li, L. et al. METTL3-mediated N6-methyladenosine modification contributes to vascular calcification. J. Mol. Cell. Cardiol. 203, 22–34 (2025).
Article CAS PubMed Google Scholar
Liu, X. et al. m(6)A-modified RIOK3 activated the NF-κB-signaling pathway by CDC42, promoting the replication and proliferation of enterovirus. Int. J. Biol. Macromol. 305 (Pt 2), 140988 (2025).
Article CAS PubMed Google Scholar
Liu, S. et al. METTL3 plays multiple functions in biological processes. Am. J. Cancer Res. 10 (6), 1631–1646 (2020).
CAS PubMed PubMed Central Google Scholar
Jiang, L. et al. METTL3-m6A-SIRT1 axis affects autophagic flux contributing to PM(2.5)-induced Inhibition of testosterone production in Leydig cells. Sci. Total Environ. 918, 170701 (2024).
Article CAS PubMed Google Scholar
Shaw, R. J. & Cantley, L. C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441 (7092), 424–430 (2006).
Article CAS PubMed ADS Google Scholar
Zhou, P. H. et al. Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis. Mediators Inflamm. 2016, 7201549 (2016).
Article PubMed PubMed Central Google Scholar
Wu, X. et al. The role and mechanism of quercetin in improving late-onset hypogonadism through network analysis and experimental validation. Naunyn Schmiedebergs Arch Pharmacol. (2025).
Liu, Y. et al. Mechanism of the traditional Chinese medicine Simiao Biejia Decoction improves the diabetes Mellitus-Induced erectile dysfunction in rats. Drug Des. Devel Ther. 19, 2609–2628 (2025).
Article PubMed PubMed Central Google Scholar
Allam, M. A. M. et al. Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J. Pharm. Pharmacol. 74 (4), 573–584 (2022).
Article PubMed Google Scholar
Abdel-Wahab, B. A. et al. Piperazine Ferulate Impact on diabetes-induced Testicular Dysfunction: Unveiling Genetic insights, MAPK/ERK/JNK pathways, and TGF-β Signaling. Naunyn Schmiedebergs Arch Pharmacol. (2024).
Badejogbin, O. C. et al. Pathogenesis of testicular dysfunction in diabetes: exploring the mechanism and therapeutic interventions. J. Assist. Reprod. Genet. 42 (2), 367–379 (2025).
Article PubMed Google Scholar
Defeudis, G. et al. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. Rev. 38 (2), e3494 (2022).
Article PubMed Google Scholar
Faselis, C. et al. Microvascular complications of type 2 diabetes mellitus. Curr. Vasc Pharmacol. 18 (2), 117–124 (2020).
Article CAS PubMed Google Scholar
Russo, V., Chen, R. & Armamento-Villareal, R. Hypogonadism, Type-2 diabetes Mellitus, and bone health: A narrative review. Front. Endocrinol. (Lausanne). 11, 607240 (2020).
Article PubMed Google Scholar
Xu, L. et al. Analysis of Differences in the Chemical Composition of Glycosides and Sugars between Four Forms of Fresh Rehmanniae Radix. Molecules https://doi.org/10.3390/molecules28247995 (2023).
Article PubMed PubMed Central Google Scholar
Contreras-Castro, A. I. et al. Chemical characterization and evaluation of the antihyperglycemic effect of lychee (Litchi chinensis Sonn.) cv. Brewster. J. Med. Food. 25 (1), 61–69 (2022).
Article CAS PubMed Google Scholar
Lei, Y. et al. Pueraria radix and its major constituents against metabolic diseases: pharmacological mechanisms and potential applications. Phytother Res. (2025).
Liu, X. et al. A Review on cryptotanshinone and its nanoformulation in cancer therapy. Anticancer Agents Med. Chem. (2025).
Ma, A. et al. The effects and underlying mechanisms of medicine and food homologous flowers on the prevention and treatment of related diseases. J. Food Biochem. 46 (12), e14430 (2022).
Article CAS PubMed Google Scholar
Okechukwu Paul-Chima, U. et al. Harnessing plant metabolic pathways for innovative diabetes management: unlocking the therapeutic potential of medicinal plants. Plant. Signal. Behav. 20 (1), 2486076 (2025).
Article PubMed PubMed Central Google Scholar
Yang, K. et al. Advances in the structural characterization and Pharmacological activity of salvia miltiorrhiza polysaccharides. Front. Chem. 13, 1492533 (2025).
Article CAS PubMed PubMed Central ADS Google Scholar
Zhou, X. et al. The Mechanism Underlying the Hypoglycemic Effect of Epimedin C on Mice with Type 2 Diabetes Mellitus Based on Proteomic Analysis. Nutrients https://doi.org/10.3390/nu16010025 (2023).
Article PubMed PubMed Central Google Scholar
Huang, H., Weng, H. & Chen, J. m(6)A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell 37(3), 270–288 (2020).
Article CAS PubMed PubMed Central Google Scholar
Yang, J. et al. N(6)-Methyladenosine METTL3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol. Ther. Nucleic Acids. 20, 111–116 (2020).
Article CAS PubMed PubMed Central Google Scholar
Zha, X. et al. Overexpression of METTL3 attenuates high-glucose induced RPE cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade through DGCR8. Aging (Albany NY). 12 (9), 8137–8150 (2020).
Article CAS PubMed Google Scholar
Chen, D. et al. The methyltransferase METTL3 regulates endothelial cell proliferation and inflammation via m(6)A RNA methylation-mediated TRAF1 expression. Biochem. Biophys. Res. Commun. 732, 150399 (2024).
Article CAS PubMed Google Scholar
Song, B. et al. Emerging role of METTL3 in inflammatory diseases: mechanisms and therapeutic applications. Front. Immunol. 14, 1221609 (2023).
Article CAS PubMed PubMed Central Google Scholar
Liu, J. Y. et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell. Death Dis. 5 (10), e1506 (2014).
Article CAS PubMed PubMed Central Google Scholar
Fu, S. et al. METTL3/YTHDC1 mediates up-regulation of LncRNA OGRU in an m6A-dependent manner involving in oxidative stress and inflammation of HG-induced Müller cells. Immunol. Lett. 272, 106972 (2025).
Article CAS PubMed Google Scholar
Liu, B. H. et al. Total flavones of Abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-Dependent m(6)A Modification-Mediated NLRP3-Inflammasome activation and PTEN/PI3K/Akt signaling. Front. Pharmacol. 12, 667644 (2021).
Article CAS PubMed PubMed Central Google Scholar
Xu, Z. et al. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell. Death Dis. 12 (1), 32 (2021).
Article CAS PubMed PubMed Central Google Scholar
Download references
We would like to thank the State Key Laboratory of Reproductive Medicine of the Nanjing Medical university for providing some experimental instruments.
This research was funded by the National Natural Science Foundation of China, grant numbers 82374459 and Collaborative Medical Research Project of Traditional Chinese and Western Medicine at Zhongda Hospital, School of Medicine, Southeast University, grant numbers 2023zxyxt14.
Wenxiu Zhang and Yuanyuan Liu contributed equally to this work.
Department of Tranditional Chinese Medicine, Qinghai Unversity Medical College Xining, Qinghai, 81006, People’s Republic of China
Wenxiu Zhang & Li Tong
School of Medicine, Southeast University, Nanjing, 210003, Jiangsu, People’s Republic of China
Yuanyuan Liu
Department of Integrative Medicine and Andrology, Zhongda Hospital, Southeast University, Nanjing, 210009, Jiangsu, People’s Republic of China
Baofang Jin & Dalin Sun
Reproductive Medicine Center, Zhongda Hospital, Southeast University, Nanjing, 210009, Jiangsu, People’s Republic of China
Yihan Jin
State Key Laboratory of Reproductive Medicine and Offspring Health, Center of Clinical Reproductive Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210006, Jiangsu, People’s Republic of China
Chao Gao & Yugui Cui
Department of Integrative Medicine and Andrology, Zhongda Hospital, Southeast University, No.87 Dingjiaqiao, Gulou District, Nanjing, 210009, Jiangsu Province, People’s Republic of China
Baofang Jin & Dalin Sun
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
PubMed Google Scholar
WZ designed the study, performed data analysis, and wrote the first draft. WZ, Y L, YJ carried out the experiments.YL, DS involved in preparing of SMBJ. DS, LT, YC and C G critically revised the manuscript. DS and BJ took the funding acquisition. The final manuscript was read and approved by all authors.
Correspondence to Baofang Jin or Dalin Sun.
The authors declare no competing interests.
All experimental procedures were approved by the Animal Ethics Committee of Southeast University (Approval No. 20200402003)in accordance with ARRIVE guidelines. This study did not involve clinical trials.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Below is the link to the electronic supplementary material.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
Zhang, W., Liu, Y., Tong, L. et al. Mechanism of the traditional Chinese medicine SMBJ alleviates diabetes mellitus-induced Leydig cell dysfunction in rats testes. Sci Rep 15, 39656 (2025). https://doi.org/10.1038/s41598-025-23251-0
Download citation
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23251-0
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
Scientific Reports (Sci Rep)
ISSN 2045-2322 (online)
© 2026 Springer Nature Limited
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.